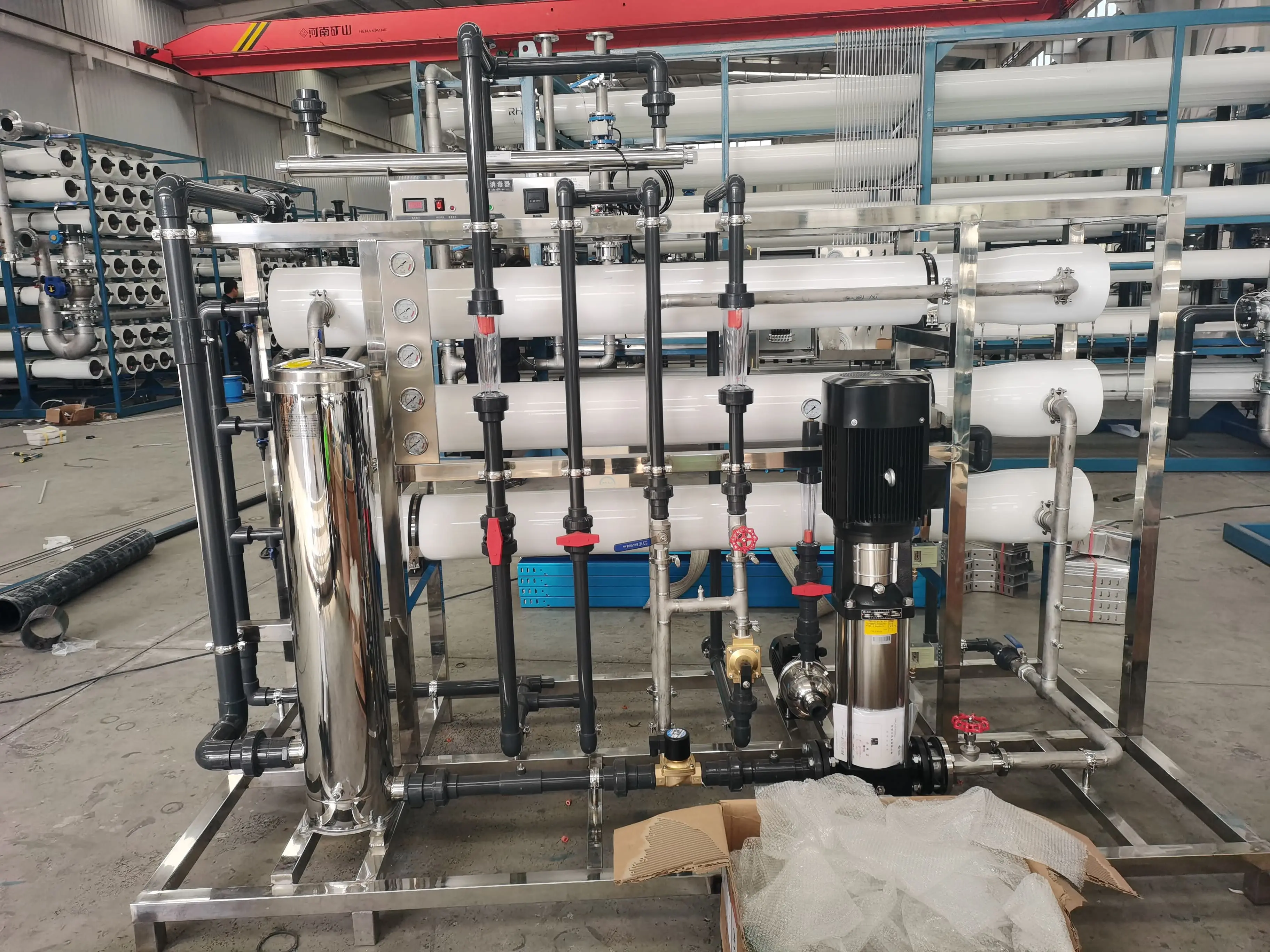
Comparison between Water Softeners and Antiscalant Dosing Devices
Softening:
Resin softening primarily involves using softening ion exchange resins to replace calcium and magnesium ions, the primary hardness ions in water, through ion exchange. This reduces the amount of calcium and magnesium ions in the water, resulting in softened water. Softened water can be used for production water with lower quality requirements, such as boiler feed water, hotel water, bathing water, central air conditioning, beauty salon water, and kitchen water. A device that uses softening water is called a Water Softener or softener. Scale inhibitors are not suitable for such systems.

Scale inhibitors:
Scale inhibitors are primarily used in reverse Osmosis Systems and become an integral part of the system through the use of a scale inhibitor dosing device. Scale inhibitors are used primarily to prevent the formation of scale-forming salts on the membrane surface, leveraging their scale inhibition and dispersing properties. These prevent scaling and clogging of the membrane by preventing calcium, magnesium, barium, and strontium ions. Proper use of reverse osmosis scale inhibitors can effectively ensure the proper operation of the reverse osmosis system and improve its performance. Therefore, this comparison shows that adding antiscalants does not conflict with resin softening. If the customer only uses water softening equipment, then antiscalants are not necessary. If the softening equipment is also part of the reverse osmosis system, then adding an antiscalant is one way to prevent RO membrane scaling and delay the precipitation of scaling substances.
The decision to add an antiscalant should be based on analyzing the scaling propensity of the water. If the water is prone to scaling, the appropriate use of an antiscalant is necessary to avoid frequent cleaning of the reverse osmosis membrane due to scaling, which can reduce membrane performance.
Comparison between them:
| Water softener | Scale inhibition system | |
| Causes of scaling | 1. Existence of hardness. 2. Exceeding the solubility of the water | |
| Scale inhibition principle | Replacing calcium and magnesium ions with sodium ions reduces hardness. Removing hardness | Using chemicals to prevent scaling and increase water solubility. Suppressing hardness |
| Disadvantages | Large investment, complex operation, high operating costs | Calcium and magnesium hardness remain in the water and require subsequent reverse osmosis removal. |
| Advantages | Removing calcium and magnesium hardness | Low investment, simple operation |
| Scope of application | Extremely high hardness Low water volume Sufficient investment Boiler and reverse osmosis industry | Large water volume Low investment Circulating water and reverse osmosis industry |