News

Working Principle and Maintenance of Reverse Osmosis Water Treatment Equipment (1)
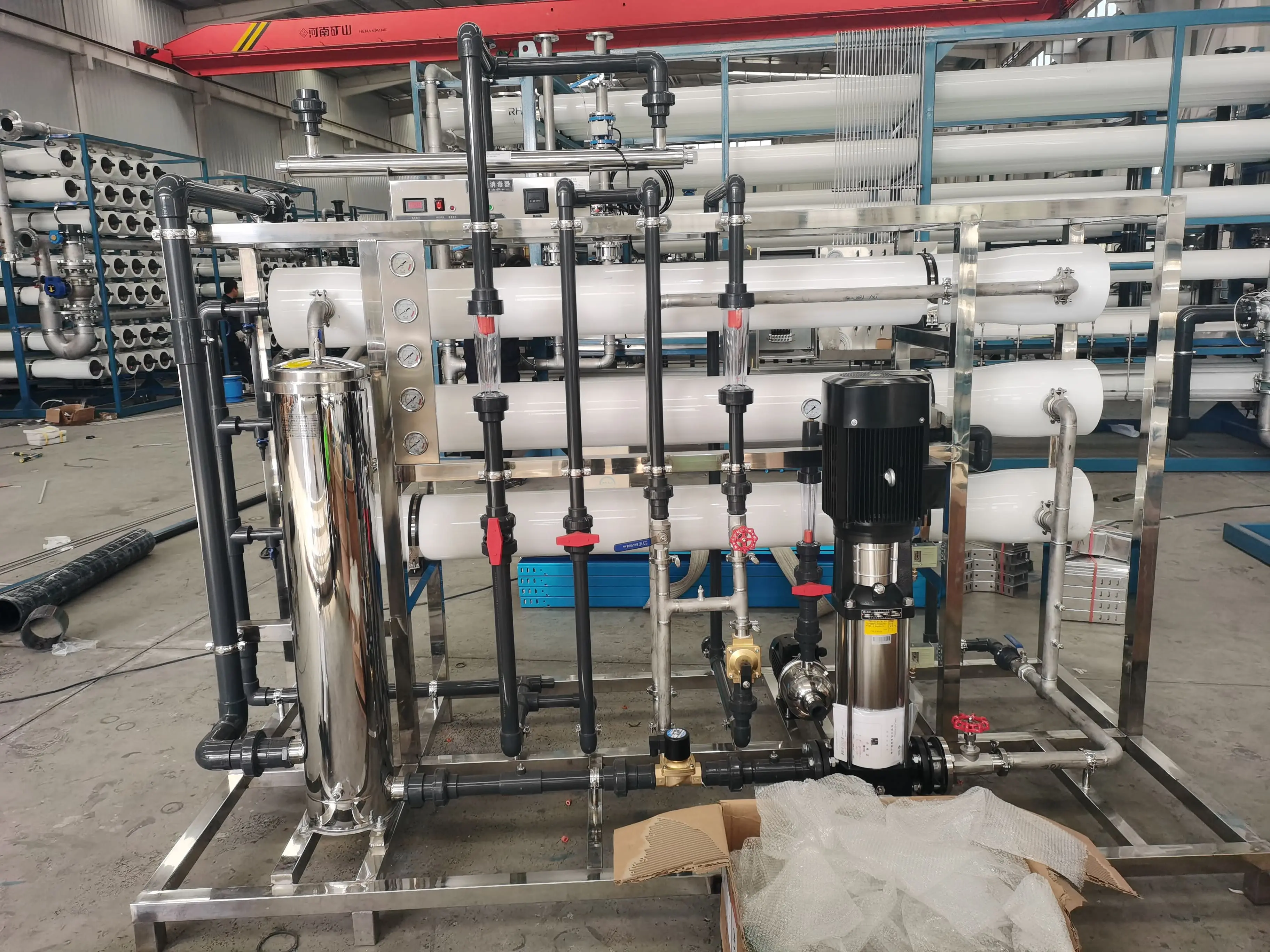
I. Equipment Principle
Reverse osmosis Water Treatment equipment typically consists of three components: a raw water pretreatment system, a reverse osmosis purification system, and an ultrapurification post-treatment system.

The Dynamic Relationship between Alkalinity and pH in Zero Wastewater Discharge
Alkalinity in wastewater is primarily contributed by three species: hydroxide (OH⁻), carbonate (CO₃²⁻), and bicarbonate (HCO₃⁻). These form a dynamically balanced buffer system, with the dominant species evolving with pH:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻ ⇌ 2H⁺ + CO₃²⁻

Why isn't the resistivity of ultrapure water infinite?
In our ideal world, "absolutely pure" water should, like a vacuum, offer infinite resistance to electric current, and its resistivity should approach infinity. However, when we measure this with the most sophisticated instruments, we find that even with today's cutting-edge technology, the resistivity of ultraPure Water remains at an extremely high, yet finite, value. The inherent properties of water and the limits of our technology dictate that there is a theoretical maximum resistivity for ultrapure water.

Factors Affecting the Fouling Rate of Surface Water Reverse Osmosis Treatment Equipment
I. Membrane Material Type (Cellulose Acetate or Polyamide)
Common reverse osmosis membranes on the market are categorized by material: CAB (Cellulose Acetate) membrane elements or CPA (Aromatic Polyamide) membrane elements. CAB membranes are often chosen over CPA membranes for difficult-to-treat surface water or wastewater systems.

Why are water hardness and alkalinity often measured as CaCO₃?
Water hardness and alkalinity, seemingly different indicators, are both typically expressed in terms of equivalent amounts of calcium carbonate (CaCO₃).
1. What are hardness and alkalinity?
Hardness: Primarily refers to the concentration of calcium ions (Ca₃⁺) and magnesium ions (Mg₃⁺) in water. These ions primarily originate from the dissolution of minerals such as limestone (primarily composed of CaCO₃) and gypsum in strata. High hardness can lead to scaling (water scale) and reduced soap efficiency.

What are the characteristics of polyaluminum chloride in wastewater treatment applications?
Polyaluminum chloride has a wide range of applications and is compatible with a wide range of water properties. It quickly forms large flocs and has excellent settling properties. Polyacrylamide molecules can form bridges and adsorb to suspended particles dispersed in the solution, resulting in a strong flocculation effect. Using the two together can achieve twice the result with half the effort. The correct combination of polyaluminum chloride and polyacrylamide is as follows:

Unqualified Boiler Softened Water and Its Treatment 2
(III) Exceeding the hardness standard for soft water in current water softening equipment is primarily caused by the following:
1. The ratio of the feed water TDS value to the resin bed height or resin exchange capacity is excessive.
Compared to the initial water test with new resin, the feed water TDS requirement for current water softening equipment is more stringent. When the resin bed height is 1.5 meters, the total hardness is 10 mmol/L, and the feed water TDS value is ≥900 mg/L, ensuring a soft water hardness of ≤0.03 mmol/L becomes more difficult.

Unqualified Boiler Softened Water and Its Treatment 1
(I) Softening Equipment Outputs Qualified Water, but Soft Water Tank Water Exceeds Standard
If the water hardness in the soft water tank exceeds the standard, but the water sampling port on the softening equipment tests qualified, this indicates that the softening equipment's water production is unstable and excessive water has been injected into the soft water tank.

Comparison between Water Softeners and Antiscalant Dosing Devices
Resin softening primarily involves using softening ion exchange resins to replace calcium and magnesium ions, the primary hardness ions in water, through ion exchange. This reduces the amount of calcium and magnesium ions in the water, resulting in softened water. Softened water can be used for production water with lower quality requirements, such as boiler feed water, hotel water, bathing water, central air conditioning, beauty salon water, and kitchen water. A device that uses softening water is called a water softener or softener. Scale inhibitors are not suitable for such systems.

Why is it necessary to remove residual chlorine before using ion exchange resins?
Before using ion exchange resins (whether for water softening, desalination, or other polishing processes), residual chlorine must be rigorously removed from the water. This is not just a standard operating procedure; it's crucial for protecting resin performance, ensuring long-term stable operation, and ensuring economic efficiency.